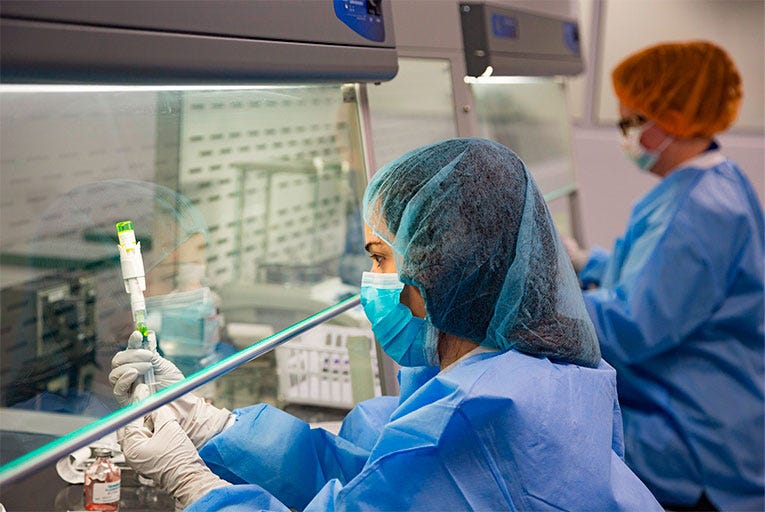
Quality
Spectrum Pharmacy Products has the right chemistry backed by quality control.
Our quality control team of more than 30 chemists and technicians perform over 20,000 tests each year in our ISO 9001-2015 in-house, certified testing facilities in California and New Jersey. This ensures safe ingredients for pharmacy products.
Our New Brunswick, NJ Corporate headquarters and site of Spectrum Pharmacy Products, contains 200,000 sq ft of packaging, laboratory and warehousing space. This facility features CFR 210/211 cGMP biopharma standard packaging capabilities and is FDA registered.
With over 125,000 sq ft of packaging, laboratory and warehousing space, our ISO 9001-2015-certified Gardena, CA facility is also FDA and DEA registered.
Meet the Quality Team


Larry Thomas
Vice President, Quality & Regulatory Affairs
Read Larry's Bio


Tom Tyner
EHS Director, Scientific & Technical Excellence
Read Tom's Bio


Tej Parikh
Compliance Manager
Read Tej's Bio


Ivan Lopez
QA Manager
Read Ivan's Bio


Robert Van Zile
QC Lab Manager
Read Robert's Bio


James Benson
QC Lab Manager
Read James' Bio
Tests routinely performed in our quality control laboratories:
Instrumental Analysis

- Atomic Absorption
- FTIR
- Fluorescence
- Gas Chromatography
- HPLC/UHPLC
- ICP/MS (Trace elemental analysis: compliance with USP <232>/<233>)
- Potentiometric Measurements (pH and ISE)
- More: Proteomic Imaging, Refractive Index, Rotational Viscosity, Specific and Optical Rotation, UV/Vis Spectroscopy, Melting Point, Karl Fischer Titration
Microbial Testing (Biopharma)

- Differentiate Bacterial Colonies
- DNase
- RNase
- Protease
- Endotoxin by LAL
- Total Plate Count
- Yeast and Mold
- Specified Organisms
Wet Chemistry

- Residue on Ignition
- Loss on Drying
- Heavy Metals
- Iodine Value
- Residue on Evaporation
- Colorimetric Analysis
- Gravimetric Analysis
- Solvent Extraction Techniques
- Titrimetry (Acidimetric, Complexometric and Redox)
Scientific Documentation - So Much More than a CofA
Spectrum offers over 600 professionally prepared dossiers on its USP chemicals available for download. This documentation is essential for pharmaceutical manufacturers, and specific products that require special testing/certification for bioburden, elemental impurities or endotoxins. Spectrum offers more than a dozen standard tests and certifications and can provide custom testing upon request. Providing such documentation on downstream production chemicals and materials used during pilot and scale-up phases mitigates manufacturer’s financial and market risk by avoiding down time, or sourcing issues as a product moves through the development process.


- Product Specification
- Safety Data Sheet
- Manufacturing Process Flowchart
- Certification of Quality Management Systems
- Product BSE/TSE Statement
- Product Allergen Statement
- Product Aflatoxin Statement
- General Label Information
- Product Certificate of Analysis Sample
- General Lot Numbering System Guidance
- Stability/Shelf Life Guidance
- Kosher and Halal Statements
Extensive Scientific Documentation and QC Support
Make Spectrum Pharmacy Products part of your Quality Management System
Customer Needs
- Eliminate unpredictability
- Reduce reworks
- Speed time to market
- Prevent product inconsistency
- Ensure reliable performance
- Streamline product onboarding
- Address formulation-related questions
- Conduct audits and review audit reports

QC Program Delivery
- Documented process and technical support to address non-conformance
- Change control, product specifications management, supply chain transparency
- QA “ticket” process to identify and address documentation or production issues among suppliers
- Lead time management
- Custom packaging
503B Outsourcing Facilities
Spectrum Pharmacy Products is backed by Spectrum Chemical to meet compliance requirements:


• FDA registered and inspected bicoastal cGMP facilities
• Certification to the ISO 9001:2015 standard
• DEA controlled substances (Schedules I-V)
• 1,200+ monograph chemical items
• 500+ in-depth scientific dossiers on monograph chemicals
• 400+ APIs covering more than 40 therapeutic areas
• 250+ top chemical excipients
• Worldwide sourcing capabilities for hard to source monographed chemicals
bioCERTIFIED™ Quality Management System & Chemicals
- Complete lot analysis including tests for endotoxin, bioburden and elemental impurities
- Comprehensive regulatory and scientific documentation
- Change control, batch traceability and supply chain transparency
- Extensive product breadth and packaging flexibility
Quality Matters: Regulatory Compliance and Auditing
Spectrum Pharmacy Products makes an ongoing effort to make compliance as easy as possible. Customers seeking to purchase sensitive, regulated or controlled items will be asked to provide appropriate credentials. For some products, we will request additional intended use information to meet regulatory or product stewardship obligations. Please help us to ship your order as soon as possible by providing required information and document expeditiously.
Spectrum Pharmacy Products hosts audits for customers. Please contact our Quality Assurance department to arrange an in-house or virtual audit.
Quality Assurance: quality_assurance@spectrumchemical.com
USA / Canada: 1-800-370-6231